Centrosomes are the major MT organising centres in animal cells and they have many important functions. Remarkably, we showed several years ago that flies could proceed through most of development without centrosomes (Basto et al., Cell, 2006), while having too many centrosomes predisposed some cells to form tumours (Basto et al., Cell, 2008). We are trying to understand how Drosophila cells cope without centrosomes, and why centrosome amplification predisposes cells to tumourigenesis, focusing in particular on the role of centrosomes in the asymmetric division of neural stem/progentior cells. We recently found that the global transcriptome is largely unperturbed in cells that lack, or have too many, centrosomes (Baumbach et al., Biology Open, 2013), and we recently discovered that centrosomes do play an important part in promoting cell cycle progression, but that this can be compensated for by the spindle assembly checkpoint protein Mad2: flies lacking centrosomes or Mad2 can proceed through most of development normally, but flies lacking both centrosomes and Mad2 fail to proliferate (unpublished observations).
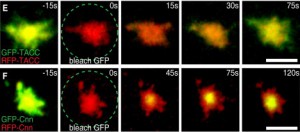
Centrosomes are formed when centrioles recruit a matrix of pericentriolar material (PCM) around themselves and we are trying hard to understand how centrioles organise the many hundreds of proteins in the PCM into a functional centrosome. In interphase, centrioles usually organise very little PCM, but, as cells enter mitosis, the PCM expands dramatically around the centrioles—a process termed centrosome maturation. Our recent studies suggest that centrosome maturation may be remarkably simple in flies. We found that the centriole protein Asl plays an important part in recruiting two proteins, DSpd-2 and Cnn, to centrioles. During mitosis, these proteins form semi-interdependent scaffolds that expand around the centrioles (Conduit et al., Curr. Biol., 2010; Conduit et al., Dev. Cell, 2014; Conduit et al., eLife, 2014) and are responsible for recruiting most, if not all, other proteins to the PCM during mitosis; in the absence of either protein centrosome maturation is reduced, but in the absence of both of them it is abolished. Intriguingly, both proteins are phosphorylated specifically at centrosomes during mitosis, and this phosphorylation is required to allow Cnn to assemble into a scaffold structure (Conduit et al., Dev. Cell, 2014). We are currently performing structural studies and combining genetics with super-resolution microscopy to understand how these proteins form scaffolds and how these scaffolds function to recruit all the other PCM components to the mitotic centrosome (Feng et al., Cell, 2017). Interestingly, mutations in Cnn have been linked to microcephaly in humans, while Spd-2 is essential for the oncogene-like induction of cellular invasion that is stimulated by centrosome amplification (Godinho et al., Nature, 2014 – a paper from the Pellman lab).